Discovery of carbon nanotubes
and their diffusion in a liquid.
Discovery of Carbon Nanotubes
In 1991, Professor Sumio Iijima of Meijo University discovered the world’s first carbon nanotubes. This remarkably stable material is 20 times stronger than steel, 1,000 times more conductive than copper, and resistant to dilute sulfuric acid and other chemicals. It quickly found its way into various applications, igniting technological innovation across diverse industries.
The battery industry took immediate notice. Many battery makers and institutions, such as GS Yuasa and Shinshu University, conducted extensive research, revealing the potential of carbon nanotubes for significant performance enhancements over lead-acid batteries. However, the industrialization of carbon nanotubes has proven challenging due to their tendency to aggregate in liquids, limiting their application in lead batteries.
Main properties of carbon nanotubes:
・High conductivity: 1,000 times more than copper
・High melting point: Over 3,000℃ (in anoxic conditions)
・Chemical stability: Stable to chemical reactions
・High corrosion resistance: Resistant to dilute sulfuric acid
・Small size: Approximately 50nm
The world’s only successful dispersion of carbon nanotubes
in a liquid.

The developer of COREDZ, worked as an engineer and was responsible for the sales department covering the western region of Japan. He managed factories in this region and visited them daily. During this time, he discovered that most factories faced significant problems and complaints related to lead-acid batteries in electric forklifts. Forklift owners were compelled to replace expensive lead-acid batteries every four years and expressed concerns about the substantial generation of lead waste. Recognizing the pressing need to refresh and extend the lifespan of lead batteries, The developer sought solutions from around the world. He subsequently came across the potential of carbon nanotubes, took on the challenge of achieving “liquid dispersion,” and successfully applied this technology for the first time in the world.
The photo on the right compares COREDZ (on the left) with similar products that use carbon nanotubes. While COREDZ can remain dispersed in liquid, other products tend to aggregate on the surface or settle at the bottom. Only COREDZ can effectively disperse carbon nanotubes in liquids, and this unique property is a critical technology for incorporating carbon nanotubes into lead-acid batteries.

Why do lead acid batteries deteriorate?
The formation of lead sulfate crystals during discharge.
Lead-acid batteries generate lead sulfate on the surface of the electrodes during discharge. When lead sulfate crystallizes, it becomes non-conductive and persists even during charging. Over repeated charge-discharge cycles, accumulated lead sulfate crystals hinder the flow of electricity within the battery, leading to reduced performance and capacity over time.

90% of lead-acid battery replacements are due to the formation of lead sulfate crystals.
10%
Physical deterioration
Main causes: vibrations and shocks, over-discharging, excessive heat, and cold.
➡Minors, COREDZ cannot refresh them.
90%
Chemical deterioration
Main cause: Sulfation (crystallization of lead sulfate on electrodes, preventing return to dilute sulfuric acid).
➡Majorities, COREDZ can refresh them.
Why can COREDZ
refresh batteries?
1
Carbon can enhance the performance of electrodes.
When carbon particles adhere to lead electrodes, they induce an electromotive force, leading to an increase in electrode voltage. Carbon particles have high conductivity, resulting in various effects such as reducing electrode resistance and facilitating the flow of electric current. This well-established scientific phenomenon has been extensively analyzed by numerous research institutions, which have published research papers on the subject.
2
Property of Carbon nanotubes
It is evident that using more conductive substances for the electrodes of lead-acid batteries could enhance their performance. However, since they use dilute sulfuric acid as the electrolyte, most metals would corrode rapidly and become unusable. While using stable materials like silver or platinum is a possibility, the cost would significantly increase, making it unaffordable for commercial sale.
In contrast, carbon nanotubes exhibit conductivity and durability surpassing that of metals, while also demonstrating corrosion resistance to dilute sulfuric acid due to their carbon composition. These properties make carbon nanotubes an excellent choice as a material for use in lead-acid batteries.
3
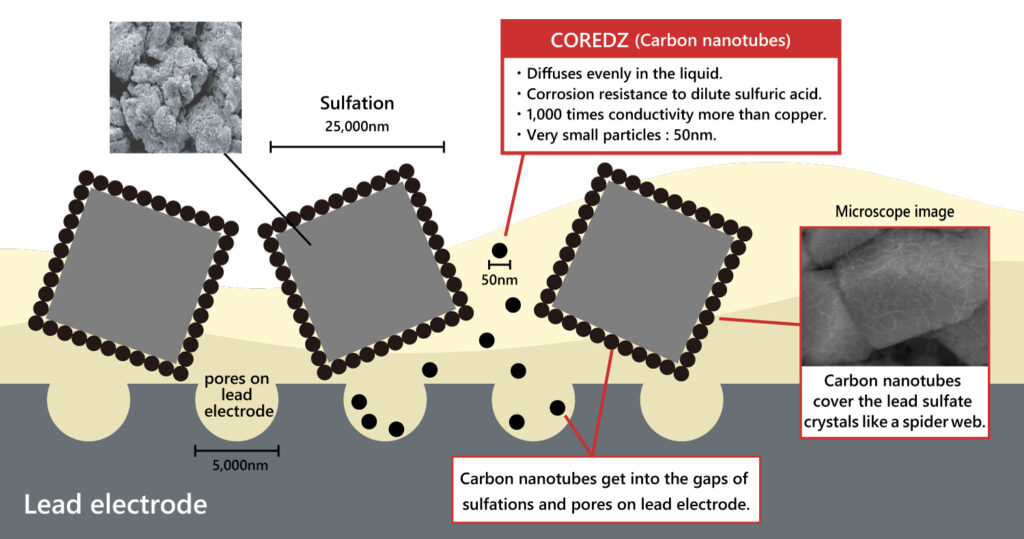
COREDZ forms conductive path
On the surface of the lead electrode, numerous lead and lead dioxide crystals have formed, along with various depressions. Additionally, a substantial number of insulating lead sulfate crystals have also developed. In comparison to these, carbon nanotubes are considerably smaller, enabling them to penetrate into the gaps and establish conductive pathways.
The conductive paths formed by carbon nanotubes exhibit conductivity 1,000 times greater than that of copper. This enables them to supply sufficient electric current to the lead sulfate crystals, which have become non-conductive. Consequently, the reduction reaction of the lead sulfate crystals becomes easier once again, transforming them into lead and lead dioxide.